Answering your burning questions about herpes simplex virus
Part 2 of “The human herpesviruses: much more than you wanted to know” Content warning: discussion of sexually transmitted disease, and one picture of oral herpes
Note: Part 1 of this series (an introduction to herpesviruses) is here.
0. If you don’t want to read this whole post, remember this actionable advice:
Talk to prospective sex partners about HSV.
Try not to contact other peoples’ saliva.
Use condoms, and consider using lube with carrageenan.
If you have HSV, take antiviral medication (e.g. acyclovir/valacyclovir).
If you’re pregnant, avoid exposure to HSV. If you have it, ask your doctor about getting a C-section.
Avoid unsanitary circumcisions (especially important if you’re Orthodox Jewish)
1. Introduction
When people hear the term “herpes”, they think of herpes simplex, a disease characterized by recurrent outbreaks of painful blisters. As the most well-known herpesviral disease, herpes simplex is also the one with the most misconceptions about it. Below are some common beliefs about this disease. By reading this post, you will learn which are true and which aren’t. (Hint: two are true, the rest are false.)
Nearly all people have herpes.
Herpes can be treated but not cured.
Herpes is just a skin condition.
Herpes is only transmitted during active outbreaks.
If someone has antibodies against herpes, it just means they were exposed, and not necessarily infected.
Condoms can reduce the risk of infection with genital herpes.
Herpes simplex is caused by two related viruses, HSV1 and HSV2. Typically HSV1 causes oral herpes and HSV2 causes genital herpes, although this is not always the case, and both viruses can infect many different parts of the body. For most people, herpes simplex is merely annoying, although severe complications can arise in some cases. This post is an in-depth exploration of HSV biology and epidemiology, and their implications for the public. Some HSV treatments will also be mentioned, but I will save most discussion of herpesvirus therapies for its own post in the future.
2. Virology of HSV
HSV1 and HSV2 are two closely related alphaherpesviruses, sharing similar biological features. HSV genomes are linear, and consist of two unique regions, each of which is surrounded by inverted repeat elements.
Source: ViralZone, Swiss Institute of Bioinformatics
The genome encodes at least 75 proteins, and more (up to 284) are being discovered as recently as 2020 (1). Clearly, there’s a lot more to learn about this. For now, I’ll describe some of the proteins that are known to be therapeutically relevant.
Surface glycoproteins gB, gD, gH, and gL interact with the surface of the target cell, and are required for viral entry (2). gB (in cooperation with gC) binds to heparan sulfate, a common polysaccharide component of the surface of human cells. Subsequently, gD, gH, and gL act to fuse the viral envelope with the target cell membrane, releasing the contents of the virus into the cell. Many vaccine candidates have targeted gD, since antibodies against this can prevent viral entry. Unfortunately, none of these candidates have yet been successful.
After entry, the capsid is transported to the nucleus and the viral genome is released. The genome is initially in a linear state, but circularizes in order for replication to take place. Replication is performed by the viral DNA polymerase, which is encoded by the genes UL30 and UL42 (3). Besides the polymerase, additional viral proteins (helicase, primase, single-strand binding protein, and origin-binding protein) are also required. The replication takes place by a “rolling-circle” mechanism, where the polymerase goes around and around the circular genome, generating a long string of concatenated linear copies which are then cleaved into individual viral genomes.
In order to produce many copies of its genome, HSV needs a lot of deoxyribonucleotides (the building blocks of DNA). Therefore, HSV encodes various proteins to use the cell’s resources to produce these. One of these, thymidine kinase (encoded by UL23) is the target of antiviral drugs. Thymidine kinase phosphorylates thymidine in order to prepare it for incorporation into DNA. The host cell also has a thymidine kinase, but the viral one has much higher activity. However, this comes at a tradeoff for lower substrate selectivity. Medicinal chemists have designed some thymidine analogs that become toxic when phosphorylated (which happens only in infected cells). This kills the cell before the virus has a chance to complete replication.
Other viral genes are important for regulation, and controlling latency vs. replication (4). VP16 is a powerful transcriptional activator that “turns on” the other genes of the virus and causes replication. This protein is actually packaged inside the viral particle, so it is present as soon as the virus enters the host cell. VP16 is a strong activator, but in order to bind to DNA it must associate with the host cell proteins OCT1 and HCF1. Since HCF1 is usually absent from the nucleus of sensory neurons, this explains why HSV tends to establish latency in these cells. During latency, no viral proteins are present. However, a viral RNA, the “latency associated transcript” (LAT) is transcribed, and this acts to suppress the other viral genes. Interestingly, deletion studies have shown that LAT is not strictly required for latency, although presumably it helps in some way to support latent infection. The process of viral reactivation is largely random, but seems to be initiated when the host cell’s RNA polymerase transcribes the VP16 gene (5). Exposure of the cell to stresses (heat, UV light, etc.), although not required for reactivation, can increase the chance that it occurs.
Besides the proteins essential for viral replication, HSV also encodes many others involved in suppressing the host cell’s immune response . ICP0 is a protein with many immune-evasion functions, including preventing apoptosis (cell suicide) and blocking the interferon response. Another example is ICP47, which inhibits the display of viral peptides on MHC receptors, preventing T cells from detecting that the cell is infected. These are only a few examples; HSV has many ways of evading the immune system, and overall this remains an area of active study.
3. Effects of HSV infection
The most common symptom of HSV is blisters, either on the lips, genitals, or more rarely on other sites of the body (6). Due to involvement of the nervous system, the blisters are often accompanied by tingling, itching, or burning sensations. HSV infections can also manifest as generalized oral inflammation or a sore throat. In general, the initial (primary) infection is more likely to have severe symptoms, due to the lack of protective antibodies. However, even primary HSV infections can be asymptomatic. During primary infections, individuals can also spread HSV to other sites on their own body, a process known as autoinoculation. This can be particularly damaging if HSV is spread to the eyes, where it can cause blindness.
Picture: a typical presentation of oral herpes as a cluster of blisters
Following primary infection, HSV spreads to sensory neurons, establishes latency, and periodically reactivates. Most reactivations result in virus shedding without overt symptoms. Typically this happens several times per month. Symptomatic reactivations can lead to painful blisters as described in the preceding paragraph. Infectious HSV is shed beginning a few days before development of blisters, and shedding persists until the blisters are healed. Often, but not always, the frequency of symptomatic reactivation gradually decreases over time.
In individuals with weak immune systems, HSV infections can be life-threatening. The most common example of this is neonatal herpes, which is usually the result of transmission by an infected mother as the baby passes through the birth canal. The risk is greatest if the mother has a primary HSV infection near the end of the pregnancy. Rates of neonatal herpes vary by population, but range from 1 in 8,200 to 1 in 1700 live births in the United States. Untreated neonatal herpes is fatal in 60% of cases, and even with treatment, survivors are significantly disabled. Neonatal herpes is also known to result from unsanitary circumcision practices in the Orthodox Jewish community (i.e. metzitzah, which is sucking the blood off) (7).
HSV can also cause life-threatening encephalitis, where the brain becomes infected. Fortunately, this is quite rare, occurring at an estimated rate of 2 – 4 cases per million people per year (8). If not treated immediately with antiviral drugs, HSV encephalitis is fatal in ~70% of cases, and survivors suffer from permanent brain damage. Even with treatment, it is fatal in 5 – 15% of cases, with 69 – 89% of survivors having long-term neurological deficits.
HSV infection has also been linked with various negative long-term effects. Genital HSV infection is associated with increased risk of infection with other STDs such as HIV (9). In my personal judgement, the studies in this area have not adequately ruled out the simple explanation that both diseases result from a shared factor of risky sexual behavior. However, genital lesions due to HSV, or increased recruitment of immune cells to the genital mucosa, may facilitate HIV infection.
Besides acute encephalitis, chronic brain infection with HSV may lead to Alzheimer’s disease, although this is not completely certain. People infected with HSV are at increased risk of Alzheimer’s disease; in particular, HSV1 primary infection or reactivation as determined by IgM antibodies was associated with a 2.55-fold increased hazard of Alzheimer’s in elderly patients (10). As determined by autopsies, HSV1 DNA is detectable in the brains of most Alzheimer’s patients, although it is also present in the brains of elderly controls. In an in vitro model, HSV1 infection of cultured neurons caused formation of amyloid-beta plaques and Tau tangles characteristic of Alzheimer’s disease (11). A large study (8362 patients over 50 years old with newly diagnosed HSV infections, and 25086 matched controls) found that the patients with newly diagnosed HSV were at a 2.56-fold increased hazard of dementia over a 10-year follow-up period (12). However, if they were given antiviral treatment, the hazard was decreased to around the same level as the control group.
While this isn’t quite a “smoking gun”, it seems reasonable that Alzheimer’s could be provoked by an immune response to infection. Recently, a trial began testing the antiviral drug valacyclovir in patients with mild Alzheimer’s disease (13). It is unclear whether HSV treatment will do anything after Alzheimer’s symptoms appear, but at least it’s more likely to work than aducanumab. (Later posts in this series will discuss the links between Alzheimer’s and other herpesviruses, such as HHV6.)
4. Detection of HSV Infections
Testing for HSV can be performed in two different ways. First, the virus can be directly detected by PCR. One set of primers you can use is ATCAACTTCGACTGGCCCTT and CCGTACATGTCGATGTTCAC, which amplifies a 179 base pair region that is present in both HSV1 and HSV2 (14). Other sequences can be used to distinguish between the two viruses.
However, PCR tests are only useful to diagnose active infections, where the virus is present in blisters. Detecting latent HSV infections by PCR would require a biopsy of the sensory neurons, which would be a highly invasive procedure. Therefore, HSV testing of people with no active symptoms is performed by analyzing the blood for antibodies. The presence of antibodies against HSV indicates the presence of a latent infection, since the immune response to HSV comes only after infection. People who say, “I have antibodies, but that just means I was exposed, not infected” are lying, unless they were enrolled in a clinical trial for an HSV vaccine (which would be unusual).
The immune response to HSV infection initially involves the production of IgM and IgA antibodies. However, the B cells that produce these antibodies soon switch to producing IgG antibodies, through a process known as isotype switching. Notably, there is a window of a few weeks between the primary infection, and when IgG antibodies become detectable in the blood. The presence of IgM antibodies can provide information about whether or not the infection was recently acquired, although IgM can also be present during subsequent outbreaks (15). IgG antibodies are what most HSV tests detect, and within a population, the prevalence of IgG antibodies is equivalent to the rate of infection.
5. HSV Epidemiology: How common is it?
Tom Lehrer describes an early example of contact tracing.
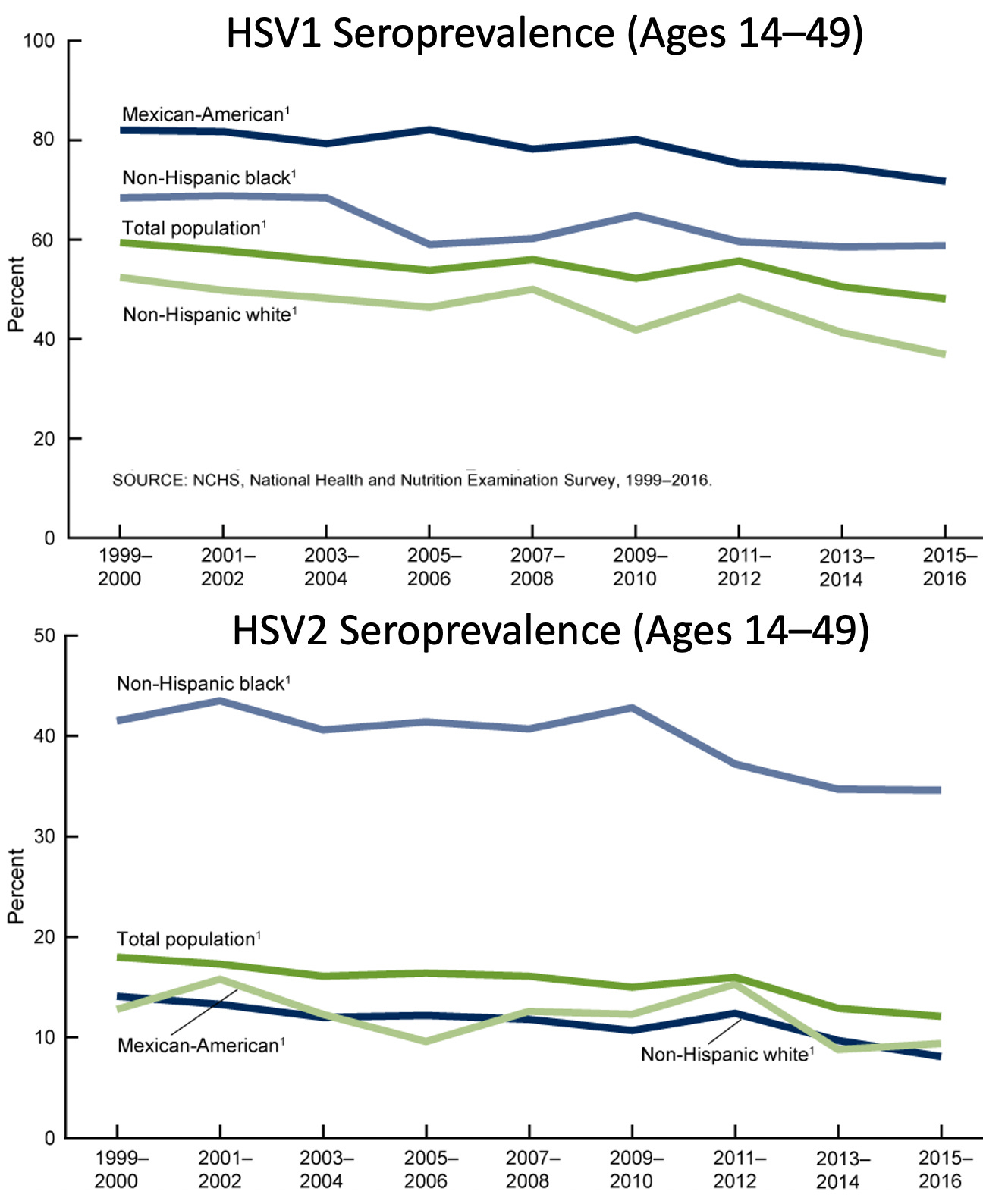
Rates of HSV infection differ between different groups of people. In general, HSV1 is more common than HSV2. The United States National Health and Nutrition Examination Survey (NHANES) has performed HSV testing on a random sample of the American population, periodically since 1999. The most recent data I could find were from the 2016 survey (16). The following graph shows rates of HSV infection by age. As expected for a lifelong infection, rates increase monotonically with age. By age 49, slightly over half of adults have HSV1, and around 20% have HSV2. The rates are slightly higher for women than for men.
United States seroprevalence in 2016 by age and sex. Red: HSV1, black: HSV2
Among young people, rates of oral HSV1 infection have declined in the United States since the 1990s, but rates of genital HSV1 infection have increased. This is likely due to the fact that oral HSV infection protects against genital infection. Rates of HSV2 infection have also decreased among young people since the 1990s, perhaps driven by safer sexual behaviors (as a response to HIV/AIDS?) or simply people having less sex. In general, Black and Hispanic minorities have increased rates of HSV infection. For HSV2, the difference is particularly striking.
Note the different axes scales for HSV1 and HSV2. Also, for some reason the NHANES switched the colors for “Mexican-American” and “non-Hispanic white” in the two graphs.
Most people with HSV do not know they have it. Either they have no symptoms, or mild symptoms which they do not recognize as herpes simplex. As part of data collection associated with a large HSV2 vaccine trial, it was found that “in asymptomatic women [ages 18–30] unaware of any HSV infection, HSV-1/-2 status was positive/negative in 45%, negative/positive in 5%, positive/positive in 7%, negative/negative in 38%, and indeterminate in 5%.” (17). Notably, these rates among women unaware of HSV infection were nearly the same as the rates in the general population.
6. HSV Transmission: Know your risks
“I swear, honey, I didn’t cheat,
I must have got it from the toilet seat.”
–An age-old lie.HSV is spread by direct contact with the skin, mucus membranes, or secretions of an infected person. HSV’s lipid envelope is relatively fragile, which means the virus cannot survive for long outside the body. Although HSV is most contagious during symptomatic outbreaks, most instances of transmission are due to asymptomatic viral shedding. A study of 498 HSV2-positive individuals showed that on average, they shed virus genitally on 18.3% of all days (18), although this varied by individual. For oral HSV1, a smaller study of 8 individuals showed that virus was shed on approximately 26% of all days (19). Clearly, the potential for transmission is present rather frequently.
The actual rates of transmission have been estimated by studies of serodiscordant couples (where one is positive and the other negative). For genital HSV, the baseline risk of transmission between such couples is approximately 5% per year, with the value depending of the frequency of sexual activity (20). Male-to-female transmission is more common than female-to-male transmission, probably due to the greater surface area of the female genitalia.
However, there is some evidence that these studies may underestimate transmission rates, since in such studies the couple knows that one of them has HSV, and so they may be more likely to avoid risky sex. The rates of transmission when the infecting partner does not know or disclose their status may be higher (21). In a study of people with new genital herpes infections, the median number of sex acts with an infected partner before HSV transmission was 40, and the median time to transmission was 60 days. These numbers were similar for HSV1 and HSV2 (both transmitted as genital infections).
Oral HSV1 infections are commonly acquired during childhood; small children spread a lot of saliva around. However, in the United States, most oral HSV infections are acquired at later ages, presumably due to kissing. Oral HSV can also be spread to the genitals during oral sex. I couldn’t find any concrete data on oral HSV transmission rates, but they are likely similar to genital HSV.
Besides sexual contact, other close skin contact can occasionally spread HSV. One example of this is herpes gladiatorum, named because it is transmitted between wrestlers. This can be caused either by HSV1 or HSV2. Historically, dentists who didn’t wear gloves were at high risk of becoming infected with HSV1 on their hands (with the potential for subsequent transmission).
There are several ways to reduce the risk of HSV transmission. Since HSV is transmitted by sex or kissing, abstaining from these activities will prevent HSV infection. This is especially important for pregnant women who, if infected, may be at risk of transmitting it to their babies. For pregnant women with HSV, delivery of the baby by Caesarean section is often recommended to avoid neonatal herpes.
Condom use is highly effective (96%) in preventing male-to-female genital transmission, and moderately effective (~65%) in preventing female-to-male transmission (22). Antiviral medication (valacyclovir) taken by HSV-positive individuals reduces the degree of viral shedding and can also prevent genital transmission, with an approximately 52% reduction in the hazard (20). This effect was on top of the reduction provided by condoms. Pre-exposure prophylaxis with valacyclovir can also prevent HSV1 transmission in the context of herpes gladiatorum (23), although surprisingly I don’t think this has been studied in the context of sexual transmission.
Various antiviral gels applied before sex can also reduce the risk of HSV transmission. Carrageenan, a common lubricant and gelling agent derived from seaweed, mimics heparan sulfate and strongly binds to HSV (as well as HPV). A 1% carrageenan gel can prevent infection of mice by HSV2 (24), although as far as I know this has not been evaluated in humans. Still, you can buy personal lubricants containing carrageenan (or else simply buy food-grade carrageenan and dissolve it in water), and since it’s very cheap and safe, there’s basically no downside to using it. Tenofovir antiviral gel, originally used to prevent HIV transmission, may also be effective in preventing HSV transmission (25), but tenofovir is harder to get than carrageenan.
7. HSV Treatments (and snake oil)
Note: Research on experimental vaccines and treatments will be discussed later in its own post.
HSV is a lifelong disease due to the establishment of latent infection, but a few treatments are available. Nucleoside antiviral drugs such as acyclovir (and its prodrug valacyclovir, designed to have better absorption when taken orally) work by killing any cells that express HSV thymidine kinase. These are effective in reducing the frequency and intensity of outbreaks, and also decrease the risk of transmission (20). However, unlike with HIV antiretrovirals, HSV antivirals do not completely suppress the disease or eliminate the risk of transmission. Resistance to acyclovir has occurred in a few cases, but is not widespread.
HSV nucleoside antivirals are generally taken orally, although there are a few formulations of topical acyclovir creams. In the United States, nucleoside antivirals are prescription-only. However, docosanol cream, which inhibits HSV fusion with target cells, is also available over-the-counter (26). In general, creams are generally less effective than oral medication because HSV reactivation begins in the nervous system, not the skin. They can reduce the duration of outbreaks, but not prevent them. Besides direct antiviral therapy, other treatments include anti-inflammatory and local anesthetic drugs.
Many sufferers of HSV take dietary supplements to try to prevent outbreaks. However, these are ineffective. I will discuss one particularly common supplement, lysine. Lysine is an amino acid present in nearly all foods at varying levels. The idea behind taking lysine supplements is that excess lysine inhibits the uptake of arginine (another amino acid) from foods, and that HSV requires arginine to replicate.
Speaking as a biochemist, this mechanism makes no sense. While HSV certainly requires arginine, this is no surprise as arginine is required for the production of most proteins. Depleting arginine to the levels required to inhibit HSV replication would have severe effects on the host. Furthermore, arginine can be produced by human cells even when it is lacking in the diet. In any case, the amount of lysine taken as supplements (generally 1 gram per day) is smaller than the amount normally in the diet, and it is unlikely that the supplements would have the desired effects on arginine levels. A literature review in 2017 found “no convincing evidence for the use of lysine to treat herpes simplex sores” (27). While a two studies found a prophylactic effect of lysine supplementation plus a low-arginine diet, the size of this effect was small, and these studies had various statistical limitations (low sample size, low compliance, etc.). In my judgment, it is unlikely that lysine supplementation is effective in treating HSV. Although there is very low risk in taking lysine, it may lead to patients not seeking more effective antiviral treatment.
8. HSV Sociology
Unlike other herpesviral diseases, most people have heard about herpes simplex, even if they don’t fully understand it. HSV, and in particular genital herpes, carries a considerable stigma in popular culture. I won’t be the judge of whether this is deserved, but the stigma certainly exists. Psychosocial consequences of symptomatic herpes simplex include anger at the infecting partner, depression, fear of rejection, and anxiety about disclosing to future partners (28). In general, individuals with a higher rate of recurrent outbreaks are more affected psychologically. Even though many people experience anger at their partner for infecting them, they may be reluctant to leave the relationship since they worry about being rejected by future partners.
While writing this post, I was given several testimonials from the members of the r/Herpes subreddit, who shared their experiences with HSV. Although these respondents are probably self-selected for more severe experiences that the overall HSV-positive population, their cases can still be useful in understanding what the disease means on a human level. I have quoted some excerpts from different respondents below.
“My physical symptoms are, luckily, more annoying than anything else, but the mental and emotional toll is excruciating. Having contracted it from someone who is asymptomatic, the fear that I'll transmit it during asymptomatic shedding is constant. This is why I don't ever kiss my children. If it weren't for the HSV group I've found and their proactive work towards awareness and supporting cure research I would be a hopeless, anxious mess.”“I just feel like I am always carrying around this secret that no one in my social group can relate to. It feels like I am alone.I also feel like I am stuck in my current relationship because my BF is the one who gave it to me and I feel if I ever were to leave the relationship no one would want me.”“Ever since contracting HSV-2 I have lost pretty much all the self-esteem I ever had. There’s always a voice in the back of my head that says “they’re going to think you’re gross because of the genital herpes”. It doesn’t help that most of the personal experiences in the partner category have been negative because of my diagnosis. 3 out of the last 4 potential partners turned me down because of my diagnosis. I know it was the diagnosis because everything was fine until we had ‘the talk’ (about HSV-2). Which I understand because I would probably make the same decision if I had the chance.”“I’m tainted, dirty and instead of an “A” on my chest (as Hester Prynne donned in the Scarlett Letter), I wear an “H” and the stigma is just as bad, maybe even worse, than being an adulterer in the 1600s. I beg those involved, the scientists, the researchers, the decision makers...please get a cure out there soon.”“It is so emotionally draining having it and feeling like a walking virus but to look for online HSV resources online and have HSV activists and influencers say "It's just a rash. Nothing to worry about" and then peddle their own products. We should not be trivializing a condition that makes 50,000 people go blind every year and that kills 500,000 babies each year also. Not saying we should shame people either but to trivialize it just makes advocating for a cure or better treatment seem so unnecessary for those who suffer worse than others from it.”On the other hand, some experiences were less negative:
“I have disclosed my status to everyone who I have slept with since I got it and they were all very sensitive and accepting. Even in times when those relationships crashed and burned, they never used my status against me to try to hurt me or say nasty things.”“Socially, I haven’t lost any friends no one treats me differently whether it be friends or family. Romantically, no one has rejected me. My dating life remained the same with me dating new and old people regularly. I have regular sex with and without condoms. They don’t treat me differently from people who are HSV negative. Physically, I’m asymptomatic so I mostly just get anxious when I think something might be a potential outbreak.”Given the stigma around HSV in context of dating, recently some dating apps for HSV positive people have been created, where users don’t have to worry about spreading HSV to a negative partner. Some examples are Positive Singles and MPWH. I don’t know how popular these apps are, but they definitely exist. Additionally, there are various support communities, such as https://www.spfpp.org/ SPFPP recently published the results of a member survey, which you can find here. They’re pretty interesting.
9. My own status
I have never had any symptoms of HSV. Although I have never been tested (standard STI testing doesn’t test for it), given my demographic information and relationship history, I am unlikely to have HSV. Although based on my search history, Google probably thinks I do.
10. A positive side to HSV: applications in biotechnology
Since this is technically a synthetic biology blog, I would be remiss if I didn’t mention the applications of HSV in biotechnology. I have personally used two genes from HSV in my research. The first, thymidine kinase, I use as a negative selection marker for cells. This is useful in two-step selection strategies, where the thymidine kinase is fused to a positive selection marker such as puromycin acetyltransferase, to form PuroTK. DNA of interest, linked to PuroTK, is introduced to cells (e.g. with CRISPR editing and HDR). Selection of edited cells is first performed with puromycin, and then a recombinase is used to remove PuroTK, leaving only the edit of interest. Any cells where PuroTK remains are then killed with a nucleoside mimic such as ganciclovir.
VP16 is also highly useful as a transcriptional activator. When fused to nuclease-inactive Cas9 (which can bind to arbitrary DNA), VP16 can activate target genes of interest. For my research, I use dCas9-VPR, containing four tandem copies of VP16 with two other transcriptional activation domains (29). This allows me to “switch on” basically any gene on-demand.
HSV itself can also be engineered for therapeutic purposes. Many researchers have tried to use HSV to kill cancer cells, and this has resulted in an approved therapy for melanoma (30), as well as several clinical trials. The basic idea is to develop strains of HSV with deletions of immune evasion genes, as well as additions of immunostimulatory genes. Delivery to cancer cells can therefore trigger an extremely powerful immune response.
In 2017, researchers published a fast and relatively straightforward method for engineering mutant strains of HSV1 (and also likely applicable to other herpesviruses) (31). The authors stated that, “While the ability to perform genome-wide editing through assembly methods in large DNA virus genomes raises dual-use concerns, we believe the incremental risks are outweighed by potential benefits.”
I strongly disagree. This paper is basically like if someone published an easy method for enriching uranium in your basement. I can only hope that nobody will misuse it.
11. Conclusions
Hopefully this post has provided all you wanted to know about HSV, and maybe a bit more. To recap, I’ll revisit some of the common beliefs about HSV:
Nearly all people have herpes.
False. In the United States, roughly 1/2 of all people have HSV1 and 1/6 have HSV2. Rates are higher in the Black population, but still not "nearly all".
Herpes can be treated but not cured.
True. Antiviral therapy can reduce, but not completely eliminate, HSV outbreaks. A true HSV cure would require eliminating all latent virus, which would be extremely challenging. However, a "functional cure" may be possible by enabling the immune system to effectively suppress reactivation. I will discuss this more in a later post.
Herpes is just a skin condition.
False. HSV infects neurons, and in rare cases can cause blindness or fatal encephalitis.
Herpes is only transmitted during active outbreaks.
False. Although HSV is most contagious during outbreaks, most instances of transmission happen when people shed HSV asymptomatically.
If someone has antibodies against herpes, it just means they were exposed, and not necessarily infected.
False. Anti-HSV antibodies are only formed in response to infection.
Condoms can reduce the risk of infection with genital herpes.
True. Condoms prevent nearly all cases of male-to-female transmission, and many cases of female-to-male transmission.
Next up: chickenpox!
12. References
1. A. W. Whisnant, C. S. Jürges, T. Hennig, E. Wyler, B. Prusty, A. J. Rutkowski, A. L’hernault, L. Djakovic, M. Göbel, K. Döring, J. Menegatti, R. Antrobus, N. J. Matheson, F. W. H. Künzig, G. Mastrobuoni, C. Bielow, S. Kempa, C. Liang, T. Dandekar, R. Zimmer, M. Landthaler, F. Grässer, P. J. Lehner, C. C. Friedel, F. Erhard, L. Dölken, Integrative functional genomics decodes herpes simplex virus 1. Nat Commun. 11, 2038 (2020).
2. R. J. Eisenberg, D. Atanasiu, T. M. Cairns, J. R. Gallagher, C. Krummenacher, G. H. Cohen, Herpes Virus Fusion and Entry: A Story with Many Characters. Viruses. 4, 800–832 (2012).
3. S. K. Weller, D. M. Coen, Herpes Simplex Viruses: Mechanisms of DNA Replication. Cold Spring Harbor Perspectives in Biology. 4, a013011–a013011 (2012).
4. M. P. Nicoll, J. T. Proença, S. Efstathiou, The molecular basis of herpes simplex virus latency. FEMS Microbiol Rev. 36, 684–705 (2012).
5. D. Fan, M. Wang, A. Cheng, R. Jia, Q. Yang, Y. Wu, D. Zhu, X. Zhao, S. Chen, M. Liu, S. Zhang, X. Ou, S. Mao, Q. Gao, D. Sun, X. Wen, Y. Liu, Y. Yu, L. Zhang, B. Tian, L. Pan, X. Chen, The Role of VP16 in the Life Cycle of Alphaherpesviruses. Front. Microbiol.11, 1910 (2020).
6. J. Esmann, The many challenges of facial herpes simplex virus infection. Journal of Antimicrobial Chemotherapy. 47, 17–27 (2001).
7. S. Tzvi-Behr, Y. Schlesinger, M. Bar-Meir MD, O. Megged, Neonatal Genital HSV-1 After Jewish Circumcision. Clin Pediatr (Phila). 55, 1245–1247 (2016).
8. M. J. Bradshaw, A. Venkatesan, Herpes Simplex Virus-1 Encephalitis in Adults: Pathophysiology, Diagnosis, and Management. Neurotherapeutics. 13, 493–508 (2016).
9. E. E. Freeman, H. A. Weiss, J. R. Glynn, P. L. Cross, J. A. Whitworth, R. J. Hayes, Herpes simplex virus 2 infection increases HIV acquisition in men and women: systematic review and meta-analysis of longitudinal studies. AIDS. 20, 73–83 (2006).
10. S. A. Harris, E. A. Harris, Herpes Simplex Virus Type 1 and Other Pathogens are Key Causative Factors in Sporadic Alzheimer’s Disease. JAD. 48, 319–353 (2015).
11. D. M. Cairns, N. Rouleau, R. N. Parker, K. G. Walsh, L. Gehrke, D. L. Kaplan, A 3D human brain–like tissue model of herpes-induced Alzheimer’s disease. Sci. Adv.6, eaay8828 (2020).
12. N.-S. Tzeng, C.-H. Chung, F.-H. Lin, C.-P. Chiang, C.-B. Yeh, S.-Y. Huang, R.-B. Lu, H.-A. Chang, Y.-C. Kao, H.-W. Yeh, W.-S. Chiang, Y.-C. Chou, C.-H. Tsao, Y.-F. Wu, W.-C. Chien, Anti-herpetic Medications and Reduced Risk of Dementia in Patients with Herpes Simplex Virus Infections—a Nationwide, Population-Based Cohort Study in Taiwan. Neurotherapeutics. 15, 417–429 (2018).
13. D. P. Devanand, Viral Hypothesis and Antiviral Treatment in Alzheimer’s Disease. Curr Neurol Neurosci Rep. 18, 55 (2018).
14. J. Stevenson, W. Hymas, D. Hillyard, Effect of Sequence Polymorphisms on Performance of Two Real-Time PCR Assays for Detection of Herpes Simplex Virus. J Clin Microbiol. 43, 2391–2398 (2005).
15. M. Hashido, T. Kawana, Herpes Simplex Virus-Specific IgM, IgA and IgG Subclass Antibody Responses in Primary and Nonprimary Genital Herpes Patients. Microbiology and Immunology. 41, 415–420 (1997).
16. H. Chemaitelly, N. Nagelkerke, R. Omori, L. J. Abu-Raddad, Characterizing herpes simplex virus type 1 and type 2 seroprevalence declines and epidemiological association in the United States. PLoS ONE. 14, e0214151 (2019).
17. J. M. Schulte, A. R. Bellamy, E. W. Hook, D. I. Bernstein, M. J. Levin, P. A. Leone, M. L. Sokol-Anderson, M. G. Ewell, P. A. Wolff, T. C. Heineman, R. B. Belshe, HSV-1 and HSV-2 seroprevalence in the united states among asymptomatic women unaware of any herpes simplex virus infection (Herpevac Trial for Women). South Med J. 107, 79–84 (2014).
18. E. Tronstein, Genital Shedding of Herpes Simplex Virus Among Symptomatic and Asymptomatic Persons With HSV-2 Infection. JAMA. 305, 1441 (2011).
19. M. Ramchandani, M. Kong, E. Tronstein, S. Selke, A. Mikhaylova, A. Magaret, M.-L. Huang, C. Johnston, L. Corey, A. Wald, Herpes Simplex Virus Type 1 Shedding in Tears and Nasal and Oral Mucosa of Healthy Adults. Sexual Trans Dis. 43, 756–760 (2016).
20. L. Corey, S. K. Tyring, R. A. Morrow, G. Mertz, M. Vargas-Cortes, Once-Daily Valacyclovir to Reduce the Risk of Transmission of Genital Herpes. The New England Journal of Medicine, 10 (2004).
21. A. Wald, E. Krantz, S. Selke, E. Lairson, R. A. Morrow, J. Zeh, Knowledge of Partners’ Genital Herpes Protects against Herpes Simplex Virus Type 2 Acquisition. The Journal of Infectious Diseases. 194, 11 (2006).
22. A. S. Magaret, A. Mujugira, J. P. Hughes, J. Lingappa, E. A. Bukusi, G. DeBruyn, S. Delany-Moretlwe, K. H. Fife, G. E. Gray, S. Kapiga, E. Karita, N. R. Mugo, H. Rees, A. Ronald, B. Vwalika, E. Were, C. Celum, A. Wald, Effect of Condom Use on Per-act HSV-2 Transmission Risk in HIV-1, HSV-2-discordant Couples. Clin Infect Dis., civ908 (2015).
23. B. J. Anderson, D. P. McGuire, M. Reed, M. Foster, Prophylactic Valacyclovir to Prevent Outbreaks of Primary Herpes Gladiatorum at a 28-Day Wrestling Camp: A 10-Year Review. Clin J Sport Med. 26, 7 (2016).
24. V. R. Zacharopoulos, D. M. Phillips, Vaginal formulations of carrageenan protect mice from herpes simplex virus infection. Clin Diagn Lab Immunol. 4, 465–468 (1997).
25. C. Celum, R. Morrow, D. Donnell, T. Hong, K. Thomas, K. Fife, E. Nakku-Joloba, A. Mujugira, J. Baeten, Sex Transm Infect, in press, doi:10.1136/sextrans-2013-051184.0823.
26. N. S. Treister, S.-B. Woo, Topical n -docosanol for management of recurrent herpes labialis. Expert Opinion on Pharmacotherapy. 11, 853–860 (2010).
27. V. J. Mailoo, S. Rampes, Lysine for Herpes Simplex Prophylaxis: A Review of the Evidence. Integrative Medicine. 16, 5 (2017).
28. A. Merin, J. E. Pachankis, The Psychological Impact of Genital Herpes Stigma. J Health Psychol. 16, 80–90 (2011).
29. A. Chavez, J. Scheiman, S. Vora, B. W. Pruitt, M. Tuttle, E. P R Iyer, S. Lin, S. Kiani, C. D. Guzman, D. J. Wiegand, D. Ter-Ovanesyan, J. L. Braff, N. Davidsohn, B. E. Housden, N. Perrimon, R. Weiss, J. Aach, J. J. Collins, G. M. Church, Highly efficient Cas9-mediated transcriptional programming. Nat Methods. 12, 326–328 (2015).
30. A. E. Bilsland, P. Spiliopoulou, T. R. J. Evans, Virotherapy: cancer gene therapy at last? F1000Res. 5, 2105 (2016).
31. L. M. Oldfield, P. Grzesik, A. A. Voorhies, N. Alperovich, D. MacMath, C. D. Najera, D. S. Chandra, S. Prasad, V. N. Noskov, M. G. Montague, R. M. Friedman, P. J. Desai, S. Vashee, Genome-wide engineering of an infectious clone of herpes simplex virus type 1 using synthetic genomics assembly methods. Proc Natl Acad Sci USA. 114, E8885–E8894 (2017).